Author: R&D Team, CUIGUAI Flavoring
Published by: Guangdong Unique Flavor Co., Ltd.
Last Updated: Feb 05, 2026

Industrial Savory Flavor Reactor
Few chemical reactions have shaped human food culture as profoundly as the Maillard reaction. From the aroma of freshly baked bread to the deep umami notes of roasted meat, the Maillard reaction is responsible for many of the savory, roasted, nutty, and complex flavor profiles that consumers instinctively associate with “deliciousness.”
For food and beverage flavor manufacturers, the Maillard reaction is not merely an academic concept—it is a controllable, designable tool. When properly understood and managed, it enables the creation of authentic savory flavors, reaction flavors, and thermal process notes that elevate soups, snacks, sauces, plant-based proteins, ready meals, and functional foods.
This article provides a technically detailed, authoritative, and application-focused exploration of the Maillard reaction, guided by Google user intent for professionals seeking practical and scientific insight. We will cover:
The goal is clear: to help flavor professionals harness the Maillard reaction with precision, consistency, and commercial success.
The Maillard reaction is a form of non-enzymatic browning that occurs when reducing sugars react with amino acids, peptides, or proteins under heat. First described by French chemist Louis-Camille Maillard in 1912, the reaction has since become one of the most studied phenomena in food chemistry.
At its core, the Maillard reaction is not a single reaction but a complex network of parallel and sequential chemical pathways, producing hundreds—sometimes thousands—of intermediate and final compounds.
According to classical food chemistry definitions, the Maillard reaction typically occurs at temperatures above 120 °C, although it can proceed more slowly at lower temperatures depending on water activity and pH. It is distinct from caramelization, which involves only sugars and no nitrogen-containing compounds.
(Source overview: Wikipedia – Maillard reaction)
Understanding the Maillard reaction requires breaking it down into three conceptual stages, each with different chemical and sensory significance.
The reaction begins when a reducing sugar (such as glucose, fructose, or lactose) reacts with a free amino group (from an amino acid like glycine or lysine).
Key events:
At this stage:
For flavor manufacturers, this stage determines reaction potential rather than immediate sensory output.
As heat exposure continues, Amadori and Heyns compounds undergo:
This stage produces many key aroma-active compounds, including:
The intermediate stage is where savory character emerges, and where industrial reaction flavors are often optimized.
In the final stage, reactive intermediates polymerize into:
Sensory impact:
From a formulation perspective, this stage must be carefully controlled to avoid:
The Maillard reaction produces an extraordinary diversity of flavor compounds. Below are some of the most commercially important classes.
Pyrazines are among the most recognizable Maillard-derived aroma compounds.
Typical sensory notes:
Common examples:
Pyrazines are especially important in:
They are potent, often active at parts-per-billion levels, making precise control essential.
Furans contribute:
Examples include:
In savory systems, furans help round harsh roasted notes, adding warmth and balance.
When sulfur-containing amino acids (cysteine, methionine) participate, the Maillard reaction produces:
These compounds deliver:
Even at extremely low concentrations, sulfur compounds dominate perception. They are foundational to meat reaction flavors, especially in:
Extensive research in food chemistry journals confirms their central role in savory aroma formation.
(Source: peer-reviewed food chemistry literature via PubMed Central)
Strecker degradation produces aldehydes such as:
These compounds act as bridges between roasted, savory, and slightly sweet notes, increasing realism in reaction flavors.
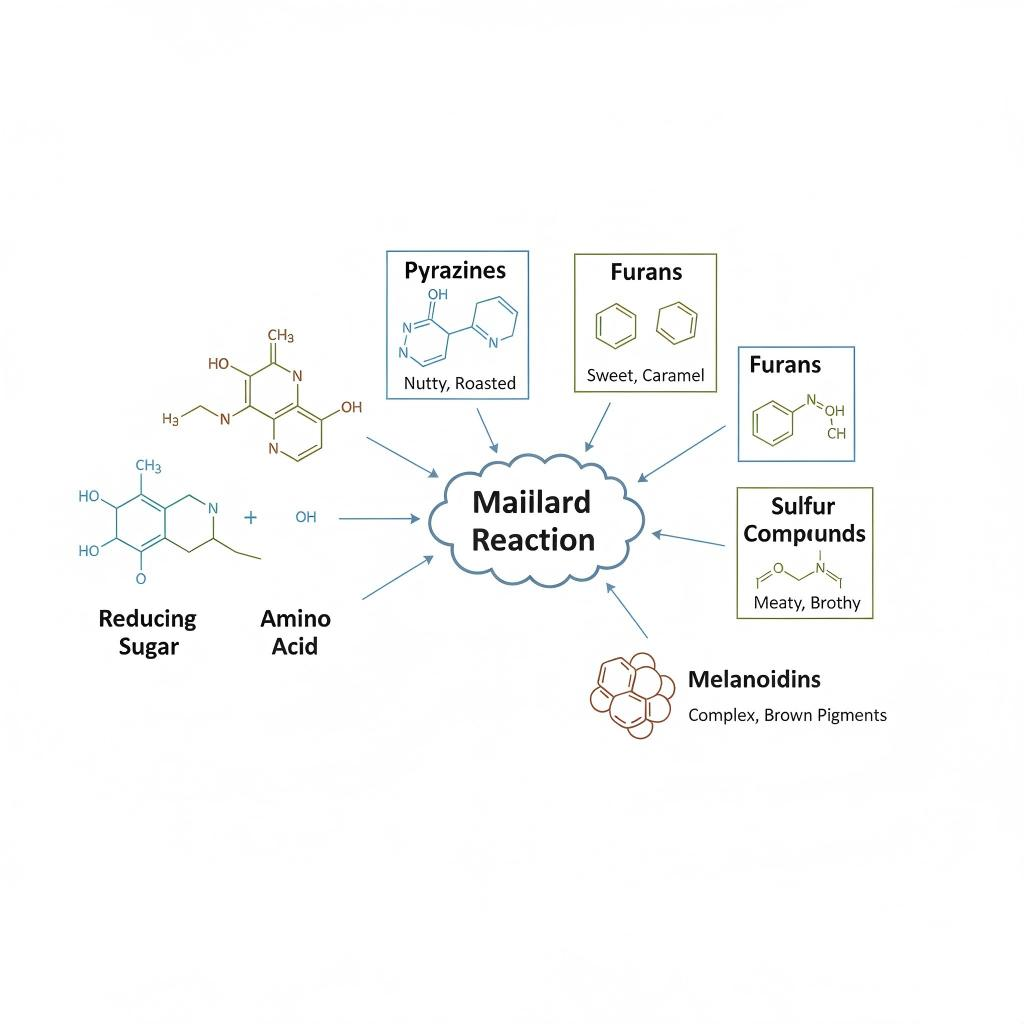
Maillard Reaction Pathway Diagram
In industrial flavor creation, the Maillard reaction is engineered, not accidental. Key parameters determine flavor direction and intensity.
Most industrial Maillard flavor reactions occur between 110–160 °C, depending on target profile.
pH has a profound effect on reaction kinetics and flavor outcome:
Careful pH buffering is essential for reproducibility.
Water activity influences:
The Maillard reaction proceeds most efficiently at intermediate water activity—too much water dilutes reactants, too little restricts mobility.
Different precursors yield dramatically different flavor outcomes:
Sugars:
Amino acids:
Selecting precursor combinations is one of the most strategic decisions in savory flavor formulation.
Maillard reaction flavors are widely used in:
They provide:
In liquid and semi-solid systems, Maillard flavors enhance:
They are particularly valuable in low-cost or reduced-meat formulations, delivering richness without excessive raw materials.
One of the fastest-growing applications is in plant-based meat analogs. Maillard reaction flavors compensate for the absence of naturally occurring meat-derived reaction products.
They help:
Major food research institutions emphasize Maillard chemistry as critical to plant-based flavor success.
(Source: university food science programs and industry reports)

Industrial Reaction Flavor Facility
Flavor manufacturers rely on advanced analytical tools:
These techniques allow chemists to link chemical composition to sensory impact.
Analytical data must be validated by sensory science:
The Maillard reaction is highly matrix-dependent—what works in a dry seasoning may behave differently in a soup or beverage base.
While the Maillard reaction creates desirable flavors, it also raises nutritional and safety considerations.
Some Maillard pathways can produce:
Regulatory agencies and food safety authorities monitor these compounds closely. The U.S. FDA and EFSA provide guidance on mitigation strategies through controlled processing.
(Source: FDA food safety guidance)
Flavor manufacturers mitigate risks by:
Maillard reaction flavors are typically classified as:
Regulatory status depends on:
Manufacturers must ensure compliance with:
Documentation and traceability are critical for global commercialization.
The future of Maillard reaction flavor creation is moving toward:
As consumer demand shifts toward plant-based, reduced-sodium, and clean-label foods, Maillard reaction flavors will play an increasingly strategic role.

Savory Food Flavor Applications
The Maillard reaction is far more than browning—it is the backbone of savory flavor chemistry. For professional flavor manufacturers, mastering this reaction enables the creation of authentic, powerful, and cost-effective savory profiles across a wide range of food and beverage applications.
By controlling precursors, reaction conditions, and delivery systems, manufacturers can consistently reproduce complex flavor signatures that resonate with consumers while meeting modern regulatory and nutritional expectations.
In an increasingly competitive food landscape, strategic use of Maillard reaction flavors is a decisive advantage.
If you are developing or reformulating savory products and would like to explore custom Maillard reaction flavors, our technical team is ready to support you.
📩 Contact us for a technical exchange or free sample request, including:
Let’s transform controlled chemistry into exceptional savory taste.
| Contact Channel | Details |
| 🌐 Website: | www.cuiguai.cn |
| 📧 Email: | info@cuiguai.com |
| ☎ Phone: | +86 0769 8838 0789 |
| 📱 WhatsApp: | +86 189 2926 7983 |
| 📍 Factory Address | Room 701, Building 3, No. 16, Binzhong South Road, Daojiao Town, Dongguan City, Guangdong Province, China |
Copyright © 2025 Guangdong Unique Flavor Co., Ltd. All Rights Reserved.